how many valence electrons in mn|Electron Configuration for Manganese (Mn, Mn2+, Mn4+) : Manila The electron configuration of manganese shows that the last shell of manganese has two electrons and the d-orbital has a total of five electrons. Therefore, . This is a patch note to a minor update / hot fix to the game, code name "Shigure Kai San". I'll keep it short. - "Mission 9 crash when restarting from checkpoint" fatal bug fixed. - We did some optimization for mission 1. The mission should have improved performance. Thank you for playing the game. PaweeMost Super Bowl squares are played on a 10x10 grid with a "home" team row on top and the "visitor" column on the side. So in this year’s matchup, the Bengals would be across the top and the Rams .
how many valence electrons in mn,Mar 23, 2023 To find the number of valence electrons for Manganese (Mn) we need to look at its electron configuration. This is necessary because Mn is a transition metal (d . The electron configuration of manganese shows that the last shell of manganese has two electrons and the d-orbital has a total of five electrons. Therefore, .Electron Configuration for Manganese (Mn, Mn2+, Mn4+)The electrons that determine valence – how an atom reacts chemically – are those with the highest energy. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. Thus, the number of valence electrons that it may have depends on the electron configuration in a simple way. For example, the electronic c.
how many valence electrons in mn Electron Configuration for Manganese (Mn, Mn2+, Mn4+) May 19, 2024
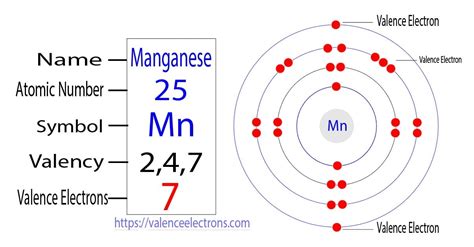
The element manganese has seven valence electrons. The electronic configuration of manganese is [Ar] 3d5 4s2. The atomic number of manganese is 25 and .

Chemical Properties. Other. The Gas Basicity of Manganese is 774.4 kJ/mol. The Dipole Polarizability of Mn is 68 plus or minus 9 a₀. Element 25 has a C6 Dispersion Coefficient .
Manganese Valence Electron Dot Diagram. An electron dot diagram is an important tool in the proper analysis of valence electrons for the element. You can also explore an in-depth electron dot diagram .Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called .
Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd .
Manganese has many isotopes, but out of these 55 Mn has the maximum abundance (almost 100%). . Periodic table with Valence Electrons Labeled (7 HD Images) Periodic table with Charges Labeled .
How many valence electrons are in one atom of each element? sulfur; helium; potassium; aluminum; Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium .

Use the group numbers to determine the number of valence electrons. The Group number of a non-transition metal can be used to find the number of valence electrons in an atom of that element. The ones place of the group number is the number of valence electrons in an atom of these elements. In other words: Group 1: 1 valence .How can you use the periodic table to find out how many valence electrons an element has? This article from Khan Academy explains the simple rules and patterns that can help you determine the number of electrons in the outermost shell of any atom. You will also learn why valence electrons are important for chemical bonding and reactivity.
Valency of Manganese is 7, 4, 2. Manganese is a transition metal, meaning that it can have more than one valence state. Its electron configuration is [Ar] 3d5 4s2. The two electrons in the 4s orbital are obvious valence electrons, so a . Manganese has 7 valence electrons. The atomic number of manganese is 25, which means it has 25 protons and 25 electrons. The first 18 electrons fill the inner energy levels, and the remaining 7 .
The element manganese has seven valence electrons. The electronic configuration of manganese is [Ar] 3d5 4s2. The atomic number of manganese is 25 and it has 25 electrons out of which seven electrons are in the last shell or orbit.
For elements in Groups 1, 2, and 12 to 18, a valence electron is an electron that has highest principal quantum number n.. Example:. For example, how many valence electrons are in arsenic?. Solution:. Arsenic is in the fourth row of the Periodic Table, so we count from left to right starting with K.From K to Ca, we are filling 4s orbitals. From Sc to .For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used. This is important as it is the Valence electrons 3d5 4s2, electrons in the outermost shell that determine the chemical properties of the element. Unabbreviated electronic configuration of neutral Manganese
The valence electron configurations of the first-row transition metals are given in Table \(\PageIndex{1}\). As we go across the row from left to right, electrons are added to the 3d subshell to .You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons, respectively. Valence electrons are responsible for the reactivity of an element.
Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first. Thus, many students find it confusing that, for example, the 5p orbitals fill immediately after the 4d, and immediately before the 6s.The filling order is based on observed experimental results, and has been confirmed by theoretical calculations.How many 3d electrons are found in the below element? manganese, Z = 25. How many valence electrons does each of the following atoms possess? a) cesium, Z = 55 c) krypton, Z = 36 b) boron, Z = 5 d) magnesium, Z = 12The electrons present in the outermost shell/orbit of an atom are called valence electrons. The valence electrons take part in any chemical reaction because the outermost orbit usually contains more energy than the electrons present in other orbits. According to the Bohr-bury scheme, the outermost orbit of an atom will have a maximum of 8 .Determine the total valence electrons (TVE) in the entire molecule (that is, the number of valence electrons of the metal plus the number of electrons from each ligand and the charge); say, it is A. Subtract this number from n . 17 electron species such as Mn(CO) .The next step is to determine how many d-electrons the Fe 3 + ion has. The rule is to count all of iron's valence electrons as d-electrons. Iron is in group 8, so. group 8 - 3+ charge = d 5 (or 3d 5) . Zinc(II) in group 12 would have 10 d-electrons in [Zn(NH 3) 4] 2+, a full shell, and manganese (VII) has zero d-electrons in MnO 4-. Nickel .how many valence electrons in mnValence electrons in the phosphate ion are 32. Example Problem 2 - Calculating Valence Electrons in a Molecule or Polyatomic Ion. Calculate the number of valence electrons in the ammonium ion {eq .
how many valence electrons in mn|Electron Configuration for Manganese (Mn, Mn2+, Mn4+)
PH0 · Valences of the Chemical Elements
PH1 · Valence electron
PH2 · Valence Electrons Chart for All Elements
PH3 · Manganese Valence Electrons
PH4 · Manganese (Mn)
PH5 · How to Find the Valence Electrons for Manganese (Mn)
PH6 · How Many Valence Electrons Does Manganese Have?
PH7 · How Many Valence Electrons Does Manganese (Mn) Have?
PH8 · Electron Configuration for Manganese (Mn, Mn2+, Mn4+)
PH9 · 3.10: Valence Electrons